Jennifer Sayer
20Nov2011
Social Media is changing the way marketers communicate with consumers. In healthcare, social media is empowering patients, facilitating physician networking, and increasing transparency through increased speed of information flows and real-time consumer reaction. This new paradigm is at significant odds with the pharmaceutical industry’s traditional approach to marketing (See Table 1), and Pharma is struggling with how best to approach the new model of manufacturer-consumer interaction afforded by social media.
Table 1: The Differences Between Traditional BioPharma Communications and Social Media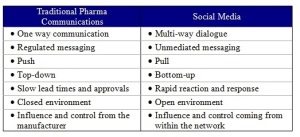
How might drug manufacturers better navigate this changing environment? Clarescent recommends several key steps for pharmaceutical companies:
- Lean towards non-branded use of social media
- Facilitate connections between and redefine key external stakeholders
- Listen continuously and respond quickly
- Build a positive corporate image with increased outward focus
Lean towards non-branded use of social media
Healthcare consumers are skeptical of pharmaceutical companies, and this healthy skepticism is evidenced in consumer advocate responses to the FDA’s call for feedback around forthcoming guidance on “Promotion of FDA Regulated Medical Products Using the Internet and Social Media Tools.” 83% of the consumer comments to the FDA were anti-pharmaceutical, and among consumer feedback was a concern that social media would be used as yet another medium for drug advertising. One consumer advocate suggested that “the last thing this country needs is more advertising by drug companies.”4
A 2009 survey of more than 2000 consumers corroborates the skepticism voiced to the FDA, revealing that over 85% of consumers would not want to use Twitter to interact with a drug company. The reasons cited for this preference include mistrust of the pharmaceutical industry as well as an aversion to the idea of being “sold to” by drug companies: ‘I don’t trust them to tell me the truth,” “They would try to sell me.” In the same survey, however, consumers note that drug manufacturers could bring value by providing disease state information, tools, and credible resources through the use of social media.
The key learning from healthcare consumers is that they are wary of drug companies and view branded information skeptically. At the same time, they have real needs around their disease that could be met through pharmaceutical company efforts. Therefore, instead of pushing branded promotion and DTC ads through social media outlets, we recommend that companies consider providing unbranded patient support through disease state education, useful tools, and references to other credible, non-branded sources of information.
Facilitate connections between and redefine key external stakeholders
Patients view patient advocates, peers, and physicians as reliable sources of healthcare information.3 While the 2009 consumer survey suggests that consumers are fearful of a social media “sell job” from drug companies, 62% of those same consumers see significant value in the use of social media tools to communicate with health care practitioners (HCPs) and experts. Whether used to consult with a physician as a means to save the time required for an office visit, to ask minor questions of a disease expert, or to network with peers to understand others’ experiences, patients view social media as a way to reach out to trusted sources to better understand their own condition. 5
Novartis’ CML Earth is a great example of how a pharmaceutical company has helped to connect key stakeholders through a unique social media platform. CML Earth is a social network site targeted to Chronic Myeloid Leukemia patients, advocacy groups, caregivers, and HCPs. Through the site, users can access an interactive map that shows the location of individual network members. Members can search for and network with other patients, caregivers, HCPs, and patient advocates – in their local areas or around the world. By hosting a network that enables key stakeholders to interact and share experiences, Novartis has used social media to meet a key need of its CML patients – that of connection.
Patients are driven to connect with peers and experts around healthcare-related issues. However, social media forums for connecting these stakeholders area not always available for specific conditions. We suggest that pharmaceutical companies provide social media tools to facilitate this connection. In doing so, companies can fulfill a significant patient need while building a positive image within the disease community.
Listen continuously and respond quickly
The case of McNeil and the social media backlash to its ads for Motrin illustrate the need for a drug manufacturer to be able to listen to social media and to respond to crises accordingly. On a Friday in September 2008, McNeil launched a campaign in magazines and online, including a “light-hearted” video they hoped would become viral. The ads were targeted to new moms who carry their babies in slings, and emphasized while that “wearing” your baby might be “in fashion” and make you feel close, the practice can be physically taxing. By the end of the day you may be sore and need Motrin.
The immediate social media response of moms to the ad was pure outrage. Thousands protested the Motrin brand on Twitter while other angry moms blogged and posted angry response videos. Countless moms pledged to never buy Motrin again. The company had not been listening and had no planned approached to dealing with the outrage, but by Sunday night responded by taking down the entire corporate web-page. By Monday, they had issued an apology that has been criticized as being stiff, clumsy, inhuman, and cold.8.9
The Motrin “Angry Moms” incident highlights the need for drug manufacturers to both listen to and respond to social media trends in real time. Companies must continuously monitor social media to retain awareness of how their brands are positioned and how consumers are responding. Further, drug manufacturers must alter internal approaches to enable rapid responses to issues fermenting in the social media space. The current copy clearance procedures within the pharmaceutical industry are not sufficient for responding to the real-time interactive reaction and dialogue afforded to consumers by social media. Moving forward, companies must change these procedures. Doing so would have enabled McNeil to better respond to the Motrin social media crisis with a voice that was human rather than corporate. This also requires a change in the organizational mind-set to being nimble and to listen.
Build a positive corporate image with increased outward focus
Studies show that healthcare consumers are distrustful of pharmaceutical companies and thus are reticent to be engaged. As such, increasing positive perception among consumers is critical for ensuring the pharmaceutical industry’s success in social media. Despite numerous social media blunders such as the McNeil Motrin example, there are a few interesting case studies for the use of social media by drug manufacturers to increase it’s standing with the public and to “humanize” it’s corporate image.
Johnson and Johnson’s BTW corporate blog, established to connect the people of Johnson and Johnson with outside stakeholders, is one such example. Launched in 2007, the company describes its blog as the voice of the company, asking “…everyone else is talking about our company, so why can’t we?” While the limitations of communication are clearly stated to consumers on the first page, the blog serves as a forum to respond directly to external questions and to address sensitive issues such as drug recalls. The blog is edited by former Bloomberg Business reporter Marc Monseau. While Monseau does most of the writing, others within the company frequently contribute, covering topics that serve to “humanize” the company such as employee activities and corporate philanthropy. Further, a blog roll on the site provides links to non-Johnson and Johnson sites and blogs that share information about health and human welfare.
While blogs such as Johnson and Johnson’s may be commonplace in other industries, BTW is unique within the pharmaceutical industry. Johnson and Johnson’s move to create a voice from within the company has been heralded as “bold and courageous” within a heavily regulated environment for which the FDA has provided scant guidance. While risky, BTW has enabled the company to humanize its corporate face and to improve its ability to communicate outwardly with consumers. It is the company brand, not the drug brand, that becomes the face to social media. Other drug manufacturers would do well to learn from their example.
References:
1. Comscore custom research, May and June 2009 (n=1002)
2. Berkman and Syme, 1979, “Social Networks, Host Resistance, and Mortality: A Nine-year Follow up Study of Alameda County Residents,” American Journal of Epidemiology 109:186-204
3. “Social Media in Healthcare,” Red Shoes PR
4. “Consumer Advocates Step Up and Submit Comments to FDA Regarding Regulation of Social Media,” Pharma Marketing Blog, March 21, 2010
5. Social media and drug companies, “What is the Value”, Rich Meyer, Online Strategic Solutions (N=2000+, 2009)
6. John George,PhiladelphiaBusiness Journal, October 10, 2008
7. Nielsen Online, “Listening to Consumers in a Highly Regulated Environment,”8/2008
8. “The Most Important Branding Investment for Pharma this Year: a Crisis Communication Plan for Social Media,” Leigh Householder, Med Ad News, 2011
9. “Offended moms get tweet revenge over Motrin ads,” USA Today, November 19, 2008
You must be logged in to post a comment.